REFERENCES
- Graham-Lomax K, Graham DY. Contemporary Diagnosis and Management of H. pylori-Associated Gastrointestinal Diseases. 3rd ed. Newtown, PA: Handbooks in Health Care Co; 2005.
- H. pylori infection. Mayo Clinic Web site. http://www.mayoclinic.com/health/h-pylori/DS00958/DSECTION=symptoms. Accessed August 30, 2013.
- Meurer LN, Bower DJ. Management of Helicobacter pylori infection. Am Fam Physician. 2002;65(7):1327-1336.
- Ables AZ, Simin I, Melton ER. Update on Helicobacter pylori treatment. Am Fam Physician. 2007;75(3):351-358.
- Helicobacter pylori. Centers for Disease Control and Prevention website. http://www.cdc.gov/ulcer/files/hpfacts.pdf. Accessed August 7, 2013.
- World Gastroenterology Organisation Global Guidelines. Helicobacter pylori in developing countries. August 2010.
- Chey WD, Wong BCY; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808-1825.
- Cardenas VM, Mulla ZD, Ortiz M, Graham DY. Iron deficiency and Helicobacter pylori infection in the United States. Am J Epidemiol. 2006;163(2):127-134.
- Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128(6):1567-1578.
- Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology. 2007;133(3):985-1001.
- Vakil N, Fendrick AM. How to test for Helicobacter pylori in 2005. Cleve Clin J Med. 2005;72(suppl 2):S8-S13.
- Chu Y-T, Wang Y-H, Wu J-J, Lei H-Y. Invasion and multiplication of Helicobacter pylori in gastric epithelial cells and implications for antibiotic resistance. Infect Immun. 2010;78(10):4157-4165.
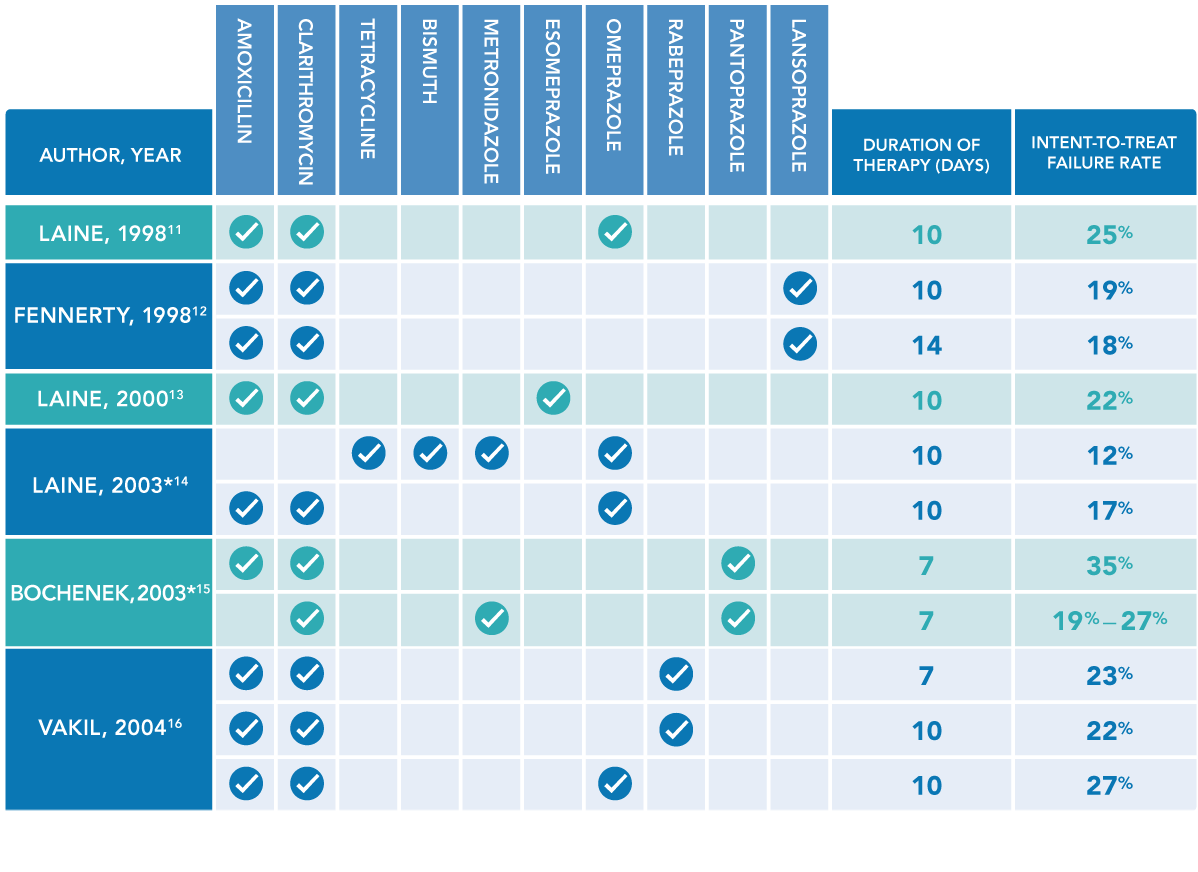
- Laine L, Suchower L, Frantz J, Connors A, Neil G. Twice-daily, 10-day triple therapy with omeprazole, amoxicillin, and clarithromycin for Helicobacter pylori eradication in duodenal ulcer disease: results of three multicenter, double-blind, United States trials. Am J Gastroenterol. 1998;93(11):2106-2112.
- Fennerty MB, Kovacs TO, Krause R, et al. A comparison of 10 and 14 days of lansoprazole triple therapy for eradication of Helicobacter pylori. Arch Intern Med. 1998;158(15):1651-1656.
- Laine L, Fennerty MB, Osato M, et al. Esomeprazole-based Helicobacter pylori eradication therapy and the effect of antibiotic resistance: results of three US multicenter, double-blind trials. Am J Gastroenterol. 2000;95(12):3393-3398.
- Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spénard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98(3):562-567.
- Bochenek WJ, Peters S, Fraga PD, et al; Helicobacter pylori Pantoprazole Eradication (HELPPE) Study Group. Eradication of Helicobacter pylori by 7-day triple-therapy regimens combining pantoprazole with clarithromycin, metronidazole, or amoxicillin in patients with peptic ulcer disease: results of two double-blind, randomized studies. Helicobacter. 2003;8(6):626-642.
- Vakil N, Lanza F, Schwartz H, Barth J. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther. 2004;20(1):99-107.
- Chiba N, Veldhuyzen Van Zanten SJ. 13C-Urea breath tests are the noninvasive method of choice for Helicobacter pylori detection. Can J Gastroenterol. 1999;13(8):681-683.
- Ore L, Hagoel L, Lavi I, Rennert G. Screening with faecal occult blood test (FOBT) for colorectal cancer: assessment of two methods that attempt to improve compliance. Eur J Cancer Prev. 2001;10(3):251-256.
- Fee C, Nemer JA. Acute infectious gastrointestinal disorders. Hosp Physician Emerg Med Board Rev Manual. 2004;8(pt 1):2-11.
- White N. Asian community at greater risk from stomach cancer; H. pylori blamed. City of Hope: Breakthroughs website. January 22, 2014. http://breakthroughs.cityofhope.org/stomach-cancer-asians.
- Malfertheiner P, Megraud F, O’Morain CA, et al; European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61(5):646-664.
- Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24(10):1587-1600.
- BreathTek UBT [prescribing information]. Otsuka America Pharmaceutical, Inc; 2013.
- American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 2005;129(5):1753-1755.
CLOSE